There is a recurring pattern in corporate gift box procurement where certified suppliers—those who have passed supplier qualification audits and hold relevant regulatory certifications—prove unable to execute approved custom designs without significant delays or specification changes. The supplier was vetted, the certifications were verified, and the design was approved, yet production stalls when it becomes clear that the customization requests fall outside the supplier's certification scope. This disconnect between supplier qualification and customization feasibility represents a critical blind spot in procurement workflows, and it stems from treating certification validation as a one-time supplier selection criterion rather than an ongoing customization approval checkpoint.
The core issue is that regulatory certifications are scope-specific and material-specific, yet procurement teams often assume that a certified supplier can execute any customization request that falls within their general product category. A supplier holding FDA food contact compliance for standard rigid board packaging is not automatically qualified to produce custom gift boxes with metallic foiling, UV coatings, or fabric inserts that will contain food products. Each customization element—every material substitution, every surface treatment, every structural modification—must be evaluated against the certification scope to determine whether additional testing, documentation, or regulatory approval is required. When this validation step is skipped or deferred until production begins, the result is predictable: project delays, cost overruns, or forced design compromises that undermine the original customization intent.
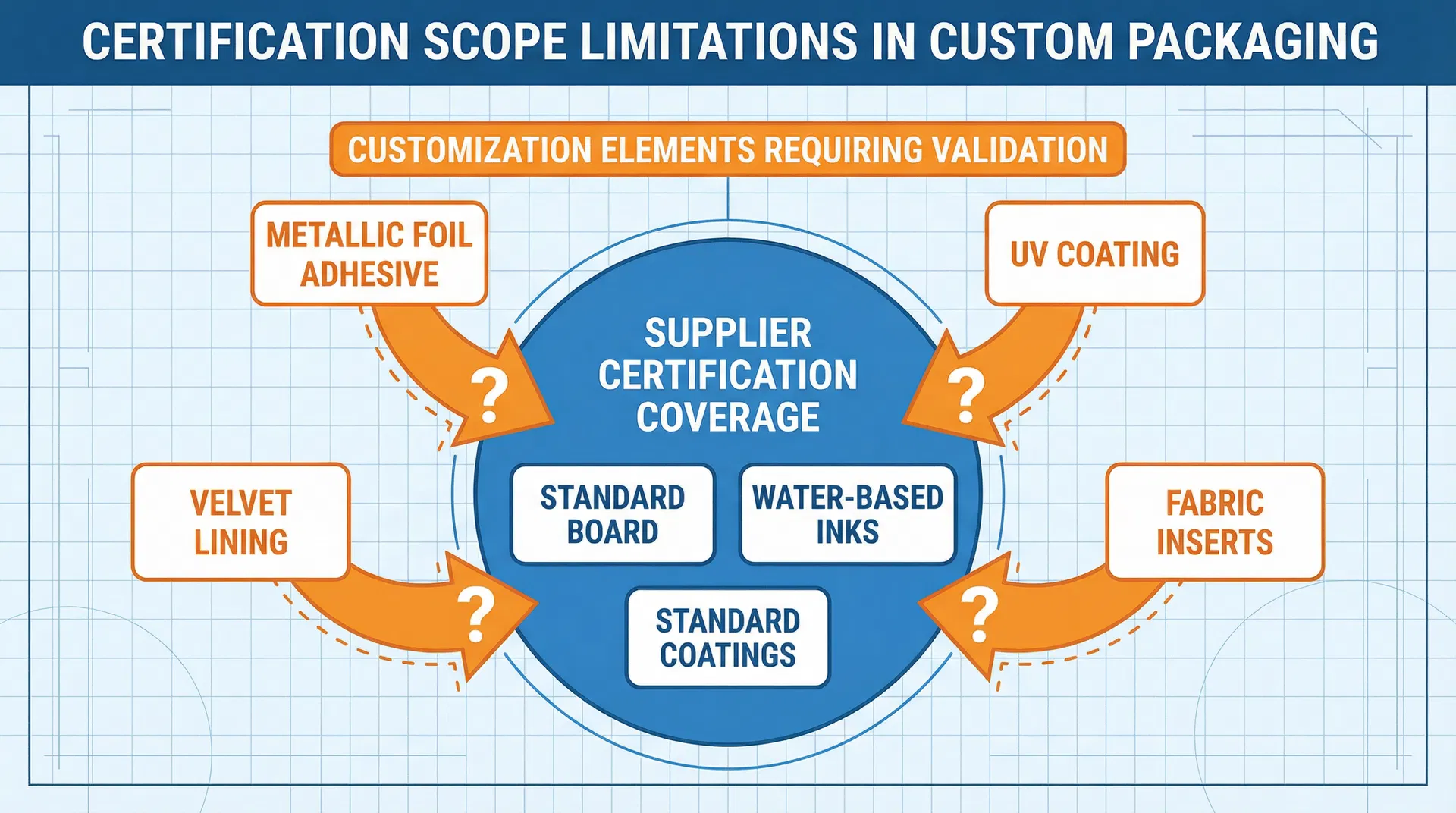
Consider the scenario of a UAE-based enterprise procuring custom gift boxes for Ramadan gifting, where the boxes will contain premium dates, chocolates, or other food items. The procurement team selects a supplier who holds FDA food contact compliance and has experience producing rigid board packaging for food applications. The design team develops a custom box featuring gold metallic foiling on the exterior, soft-touch lamination for tactile appeal, and a velvet-lined interior tray to cradle the food products. The design is approved internally, the supplier confirms feasibility, and production is scheduled to begin. Two weeks into the timeline, the supplier informs the procurement team that the specific metallic foil adhesive used in their standard process has not been tested for food contact migration, and the velvet lining material requires biocompatibility documentation that the supplier does not currently possess. The project is now delayed by three to four weeks while the supplier arranges migration testing for the foil adhesive and sources alternative lining materials with existing food contact certifications—or the design must be modified to eliminate the elements that triggered the certification gap.
This scenario is not an edge case; it reflects a systematic failure to validate certification scope during the customization approval process. The supplier's FDA food contact compliance covered their standard packaging materials—the base board, the standard printing inks, the water-based coatings—but it did not extend to the custom elements that differentiated this project from their routine production. The procurement team assumed that certification was binary: either the supplier is certified or they are not. In reality, certification is conditional: it applies to specific materials, specific processes, and specific end-use applications, and any deviation from those parameters requires re-validation.
The problem is compounded by the fact that certification scope limitations are not always visible during supplier qualification or design approval. Suppliers may hold broad certifications—ISO 9001 for quality management, BRCGS for food safety systems—that signal operational competence but do not guarantee that every material or process they use is covered under those certifications. A supplier with BRCGS certification operates under audited food safety protocols, but that does not mean every ink, adhesive, or coating in their facility has been tested for food contact compliance. When procurement teams request customization, they are often introducing materials or processes that the supplier has not previously used in food-contact applications, and the certification gap only becomes apparent when the supplier's quality assurance team reviews the production specifications.
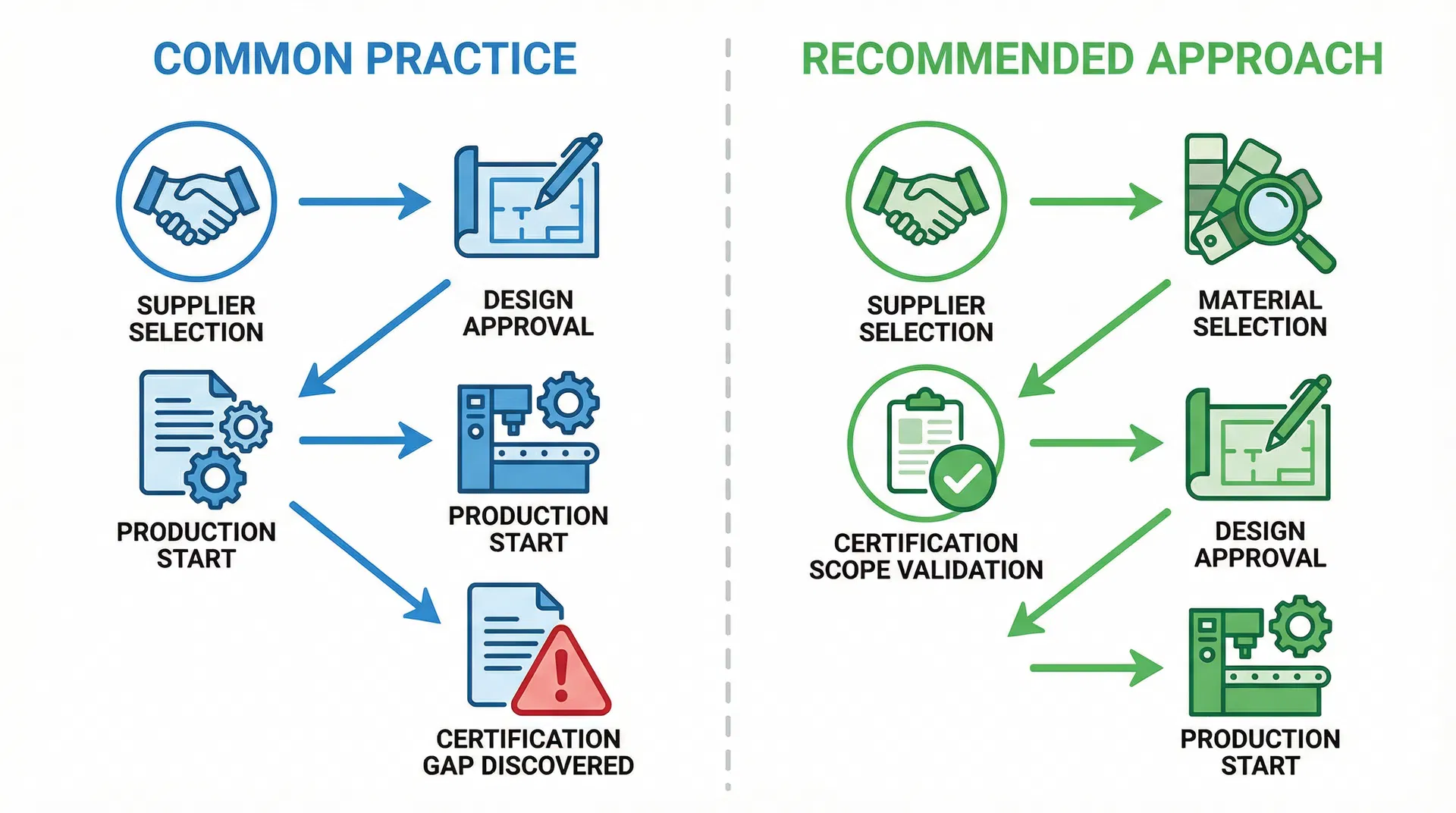
In practice, this is often where decisions about custom corporate gift box specifications begin to be misjudged. Teams approve customization requests based on aesthetic appeal and brand alignment, without systematically evaluating whether those requests introduce materials or processes that fall outside the supplier's existing certification coverage. The approval workflow focuses on visual design, structural feasibility, and cost—but it does not include a certification scope validation step where each customization element is cross-referenced against the supplier's regulatory documentation to confirm that no additional testing or approval is required.
The regulatory landscape adds further complexity, particularly for enterprises operating across multiple markets. A custom gift box designed for UAE distribution may need to comply with UAE food safety regulations if it contains food items, but if the same box is intended for export to EU markets, it must also meet EU food contact material regulations, which impose different testing requirements and migration limits. A supplier certified under FDA standards may not hold the equivalent EU certifications, and customization choices that are acceptable under FDA guidelines may not comply with EU requirements. Procurement teams that fail to validate certification scope against all relevant regulatory jurisdictions risk discovering compliance gaps only after production has begun, when rectification options are limited and costly.
The material substitution risk is particularly acute in custom gift box production, where suppliers may propose alternative materials to meet cost or lead time targets without fully disclosing the certification implications. A supplier might suggest replacing a certified food-contact board with a visually identical alternative that costs 15% less, without mentioning that the alternative board has not been tested for food contact compliance. Or they might propose a different foil supplier to reduce lead time, without verifying that the new foil's adhesive system has the same food-safe certifications as the original. These substitutions are often presented as minor production optimizations, but they can invalidate the certification coverage that the procurement team relied upon during supplier selection.
For UAE-based corporate gifting programs, where gift boxes frequently contain premium food products—dates, chocolates, nuts, confectionery—the certification scope issue carries significant compliance and reputational risk. If a custom gift box is distributed to VIP clients or government officials and later found to contain non-compliant packaging materials, the consequences extend beyond regulatory penalties to include reputational damage and relationship strain with high-value stakeholders. The UAE market's emphasis on quality and compliance leaves little tolerance for packaging that fails to meet food safety standards, particularly when the gifting company is a multinational corporation subject to both local and international regulatory oversight.
The practical response is to integrate certification scope validation into the customization approval workflow, treating it as a mandatory checkpoint rather than an optional supplier qualification step. This means requiring suppliers to provide material-specific certification documentation for every customization element—not just general certifications like ISO 9001 or BRCGS, but specific compliance statements for the inks, adhesives, coatings, and substrates that will be used in the custom design. It means asking suppliers to confirm, in writing, that all proposed materials fall within their existing certification scope, or to identify which elements require additional testing and what the associated timeline and cost implications are. And it means conducting this validation before design approval, not after production has begun, so that certification gaps can be addressed through design modifications or supplier changes rather than through costly mid-production delays.
The broader principle is that certification is not a static supplier attribute—it is a dynamic constraint that must be re-evaluated every time customization introduces new materials or processes. A certified supplier is only certified for the specific materials and processes covered by their documentation, and any deviation from those parameters requires explicit validation. Understanding this distinction, and building certification scope validation into the customization approval process, is essential for procurement teams who want to avoid the delays, cost overruns, and compliance risks that arise when customization requests exceed supplier certification coverage.
Related Articles
Customization Process for Corporate Gift Boxes
Comprehensive guide to navigating the customization process for premium corporate gift packaging in the UAE market.
Prototype Approval vs Production Functionality
Why structurally approved custom gift boxes often fail at scale and how to evaluate designs for production viability.